Life Sciences:Product Recall Trends and Risk Mitigation Strategies
Jun 17, 2025
Medical device recalls have increased significantly, with 2024 witnessing a notable rise in recall events and impacted units. Design-related and manufacturing defects are the leading causes of device recalls, while Class I recalls, posing the most serious safety risks, have also surged. The FDA is enhancing the recall process and communication, including a pilot program for early alerts about potentially high-risk devices. This article examines FDA product recall trends for medical devices and pharmaceutical companies, highlighting key statistics and industry implications. It also explores the effects of supply chain issues on recalls and provides best practices and risk management tragedies in the event of a recall.
While 2021 saw a noticeable decrease in overall recall events, 2024 has reached almost similar numbers. If the current trajectory continues, 2025 will likely see a comparable or higher number of recalls, considering data through April and eight months remaining.
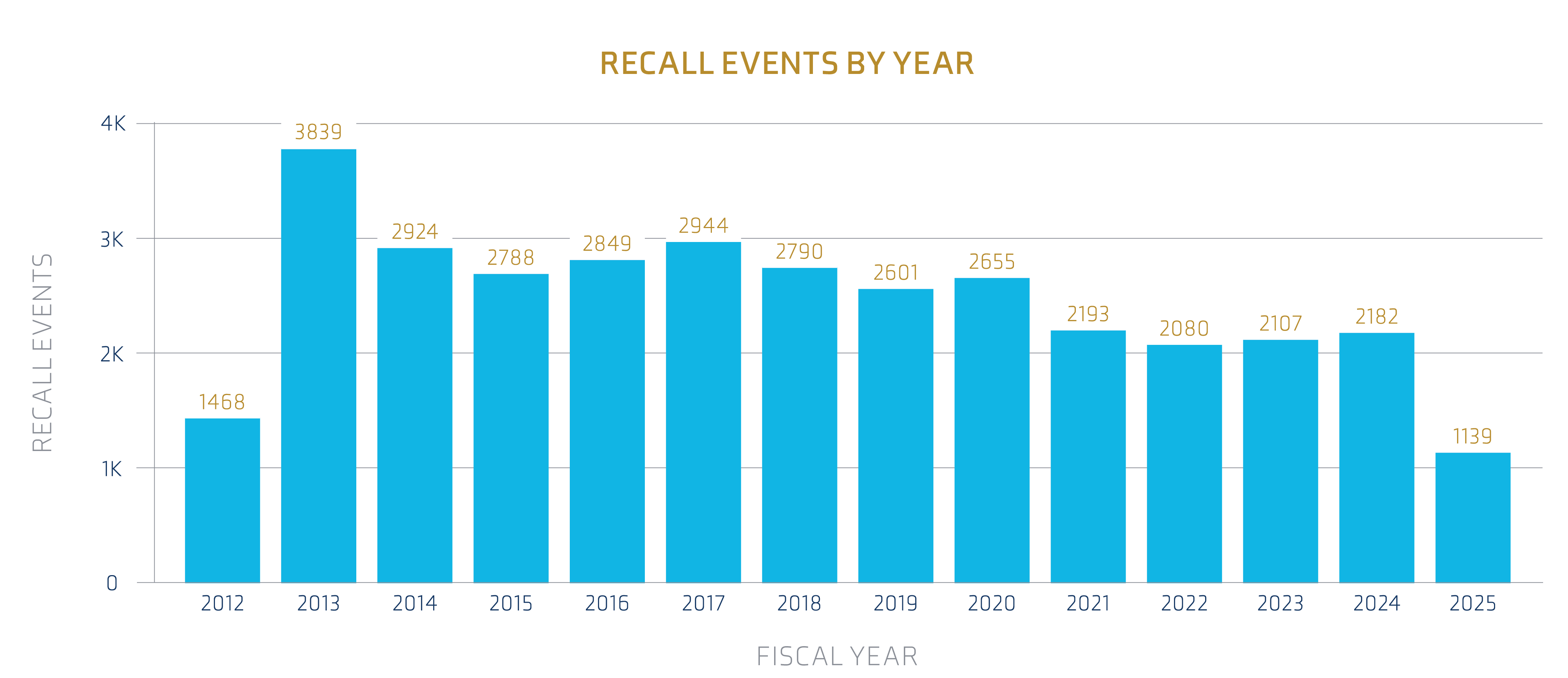
Source: FDA Recall Events Database1
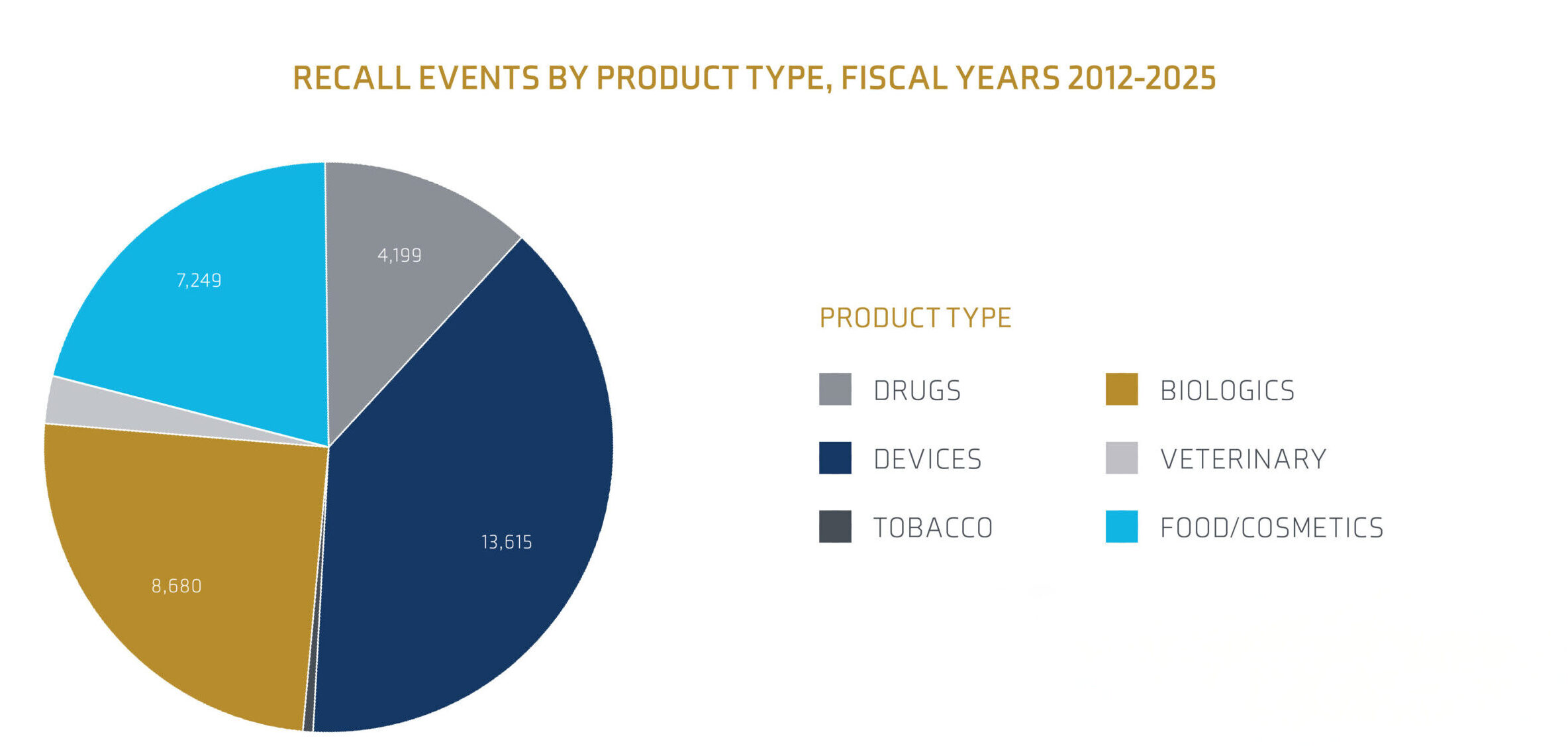
Source: FDA Recall Events Database1
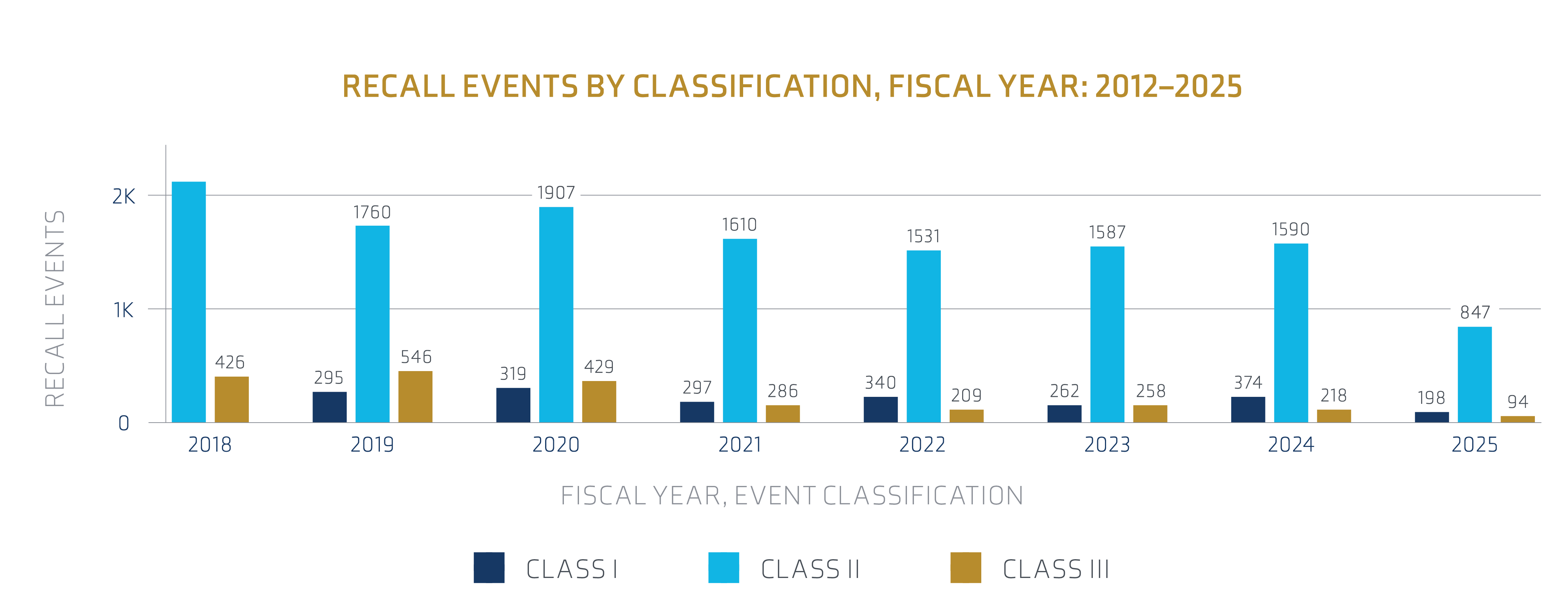
Source: FDA Recall Events Database1
Medical device recall events have been trending upwards, significantly increasing in 2024 to a four-year high. Notably, Class I recall events, which indicate a reasonable probability of causing serious adverse events or death, are at a 15-year high. According to FDA data, medical device recalls fluctuated over the past decade, with design-related issues, manufacturing defects and device failure being the leading causes. The rise in recalls can be partly attributed to the rapid development and deployment of new medical technologies, which sometimes encounter unforeseen issues. Software problems play a significant role in medical device recalls.
Source: FDA Recall Events Database1
Class II recalls, indicating moderate risk issues, dominate the pharmaceutical sector. While the number of Class I recalls remains relatively stable, they continue to highlight severe drug safety concerns. Increased regulatory scrutiny and more robust reporting mechanisms have contributed to identifying and recalling potentially harmful pharmaceutical products.
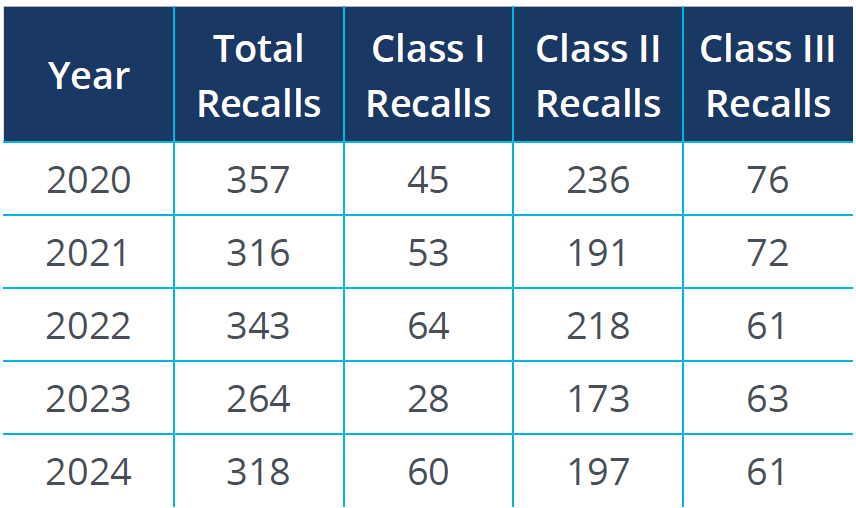
Source: FDA Recall Events Database1
The trends in FDA recalls emphasize the need for stringent regulatory compliance for medical devices and pharmaceutical companies. Organizations must invest in robust quality control systems and proactive monitoring to detect and address potential safety issues early.
While innovation drives new treatments and technologies, it also poses safety and efficacy challenges. Companies must balance innovation with comprehensive risk management strategies to ensure patient safety.
Frequent recalls can affect consumer confidence in medical products. Transparent communication and swift action in addressing recalls are essential for maintaining public trust.
The supply chain is crucial in the manufacturing and distribution of medical devices and pharmaceutical products. Disruptions and inefficiencies within the supply chain can significantly impact product quality and safety, leading to an increase in product recalls.
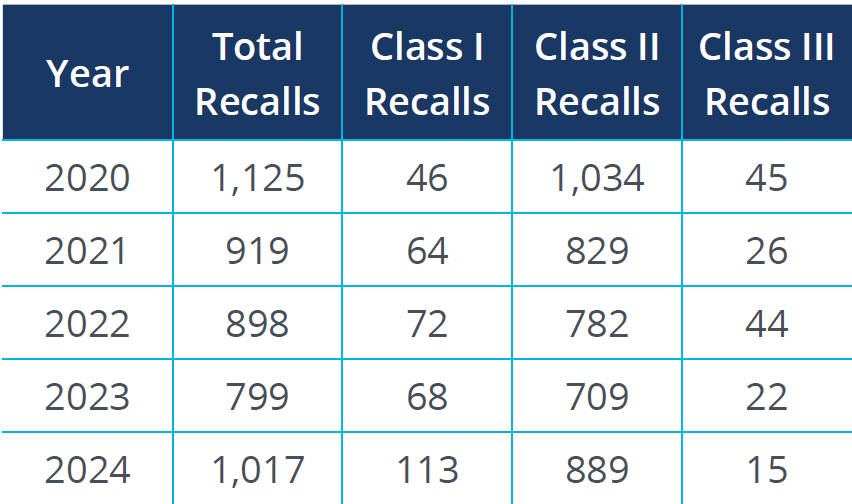
The following table illustrates the correlation between supply chain disruptions and medical device recalls over recent years:
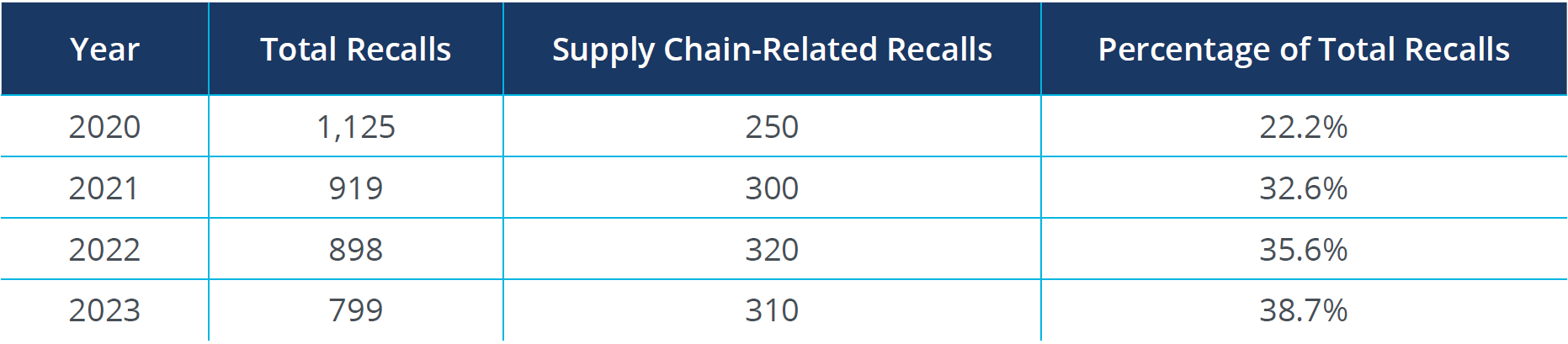
Source: FDA Recall Events Database1
The impact of supply chain issues on pharmaceutical recalls is significantly greater than in the medical device sector:
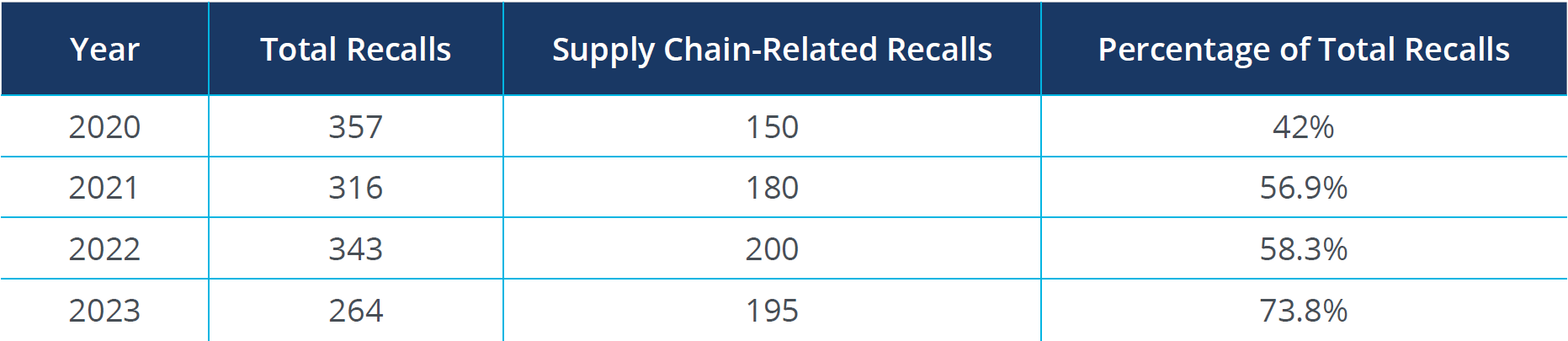
Source: FDA Recall Events Database1
The percentage of recalls attributed to supply chain issues has steadily increased, highlighting the growing impact of supply chain disruptions. Inconsistent quality control across the supply chain is a major contributor to recalls, emphasizing the need for robust quality assurance mechanisms. Navigating different regulatory standards across countries can lead to compliance issues, increasing the risk of recalls.
The modern supply chain is highly complex and often spans multiple countries and continents. This globalization introduces several challenges:
Many companies adopt just-in-time (JIT) manufacturing to reduce inventory costs. While JIT can improve efficiency, it also increases vulnerability to disruptions:
Shortages of raw materials can lead to compromises in product quality:
The best time to prepare for a recall is before an event occurs. There are several strategies and measures life sciences companies can implement to reduce the likelihood of a recall and help mitigate the impact of a recall event when it occurs.
Building strong relationships with reliable suppliers can mitigate supply chain risks:
Diversification can reduce dependence on a single supplier or region:
Advanced technologies can enhance supply chain visibility and efficiency:
Product recall coverage is typically excluded from all life sciences product liability and errors and omissions liability insurance policies. Still, it may offer a sub-limit to cover first-party expenses related to a recall.
When utilizing contract manufacturing organizations (CMOs) to produce products, having strong contractual controls is a first defense. All CMOs should be required to carry minimum limits of insurance, list the contracted party as an additional insured on the CMO’s policy, and contractually require the CMO to indemnify the contracted party for manufacturing errors and omissions and related recall costs and expenses.
Product recalls are inevitable in the life sciences industry to ensure patient safety and public health. The FDA has consistently emphasized that firms must be “recall ready.”
As regulatory pressures intensify, it is crucial for manufacturers to assess how prepared they are to withstand a product incident or crisis:
A formal written recall plan should address and describe the following procedures:
A mock recall is a simulated process to test and evaluate the effectiveness of a formal recall plan. Mock recalls can help identify vulnerabilities in traceability or faults in a recall strategy. Mock recalls are a mechanism to improve procedures and increase readiness to ensure swift action in the event of a genuine recall.
The supply chain is a critical factor influencing medical device and pharmaceutical product recalls. Addressing supply chain challenges through strategic supplier relationships, diversification, and technological investment can significantly reduce recall risks and enhance product quality. Proactively managing supply chain risks helps protect public health and maintain regulatory compliance.
Implementing risk mitigation strategies to reduce recall likelihood is essential. Additionally, having a robust and tested recall program minimizes potential impacts in the event of a recall.